The Truth About Pharma Reps Sending Text Messages to Healthcare Professionals

by Kimberly Gregorio | Last Updated: October 26, 2023 | 1 min read
The debate around whether pharmaceutical reps can or should exchange text messages with healthcare professionals (HCPs) is becoming increasingly complex. Some within the industry feel that texting with HCPs increases the risk of noncompliance. However, as technology continues to evolve, those concerns are becoming a thing of the past.
Today, compliance-enabled texting with HCPs is possible. And in many cases, it’s preferred. But that doesn’t mean pharmaceutical teams can forget the rules and regulations around communicating with HCPs. Quite the opposite.
While technology can help better enable compliance measures, reps still need to understand what they are up against.
Understanding the Compliance Conundrum
Navigating the regulatory landscape, which includes laws like the FCC's TCPA, FTC's CAN-SPAM Act, HIPAA, FCPA and the DOJ's ECCP, can be daunting. However, despite this, pharmaceutical sales teams must try to fully understand how these rules and regulations apply to their communications practices. Adhering to these regulations not only helps companies maintain their reputations, it can also help them avoid fines and other penalties.
For example, in a recent update to the Evaluation of Corporate Compliance Programs (ECCP), the DOJ's Criminal Division has introduced important changes that pharmaceutical companies must be aware of. One revision involves personal devices, communication platforms and messaging applications. Sales teams often communicate with HCPs using personal devices, and the new guidance stresses the need for companies to have policies and procedures in place to retain all business-related communications. It also emphasizes the importance of ensuring employees do not use unapproved applications and devices for business purposes.
Another notable revision to the ECCP provides expanded guidance on how compensation structures can drive compliance. This includes using financial penalties, such as compensation clawback provisions, to discourage non-compliant behavior. The guidance urges companies to establish a strong connection between compliance and compensation, meaning that compensation plans should reward employees who demonstrate strong ethical compliance and penalize those who do not. Pharmaceutical sales teams must know these compensation structures and ensure their actions align with company policies and ethics.

Explore More Relevant Articles on P360
- P360 Presents ZING During the Diversity Alliance for Science’s (DA4S) East Coast Conference
- The Art and Science of Omnichannel Marketing
- Why Pharmaceutical Companies Need to Prioritize Customer Experience (CX) Initiatives
- Say No to Apps, Portals and Logins for Effective Physician Engagement
- ZING Powers HCP Engagement With Intelligent Bots and QR Codes
What Pharma Sales Teams Can Do
With the increasing use of personal devices and text messaging, pharmaceutical sales teams must take steps to ensure that their messaging practices remain compliant. The rise of instant communication channels has made it easy for reps to initiate inappropriate conversations with clients, employees or anyone within their network. The updated ECCP provides recommendations on the best practices regarding using personal devices for business, such as implementing security measures to prevent noncompliant behavior. Organizations can leverage this guidance by providing clear policies that ensure employees adhere to the necessary protocols.
One way to do that is to prioritize compliance training. This requirement not only helps teams understand the boundaries of regulatory compliance but also informs them on how to avoid violations effectively. And best of all, training helps reduce a company’s exposure to legal and ethical risks.
Organizations should also establish clear communication guidelines to ensure that all text messages remain compliant. These guidelines can include details such as appropriate messaging content, limitations of message content and appropriate parties who can initiate messages with HCPs. Organizations can also implement measures that ensure all text messages are reviewed and approved before being sent to HCPs. And the best way to do that is to leverage technology.
But What Technology Should Pharma Teams Use?
The technology platform for leading Pharma brands is the ZING Engagement Suite. ZING supports Pharma-to-HCP text messaging in a way that addresses today’s complex compliance issues. Every message sent through ZING can be preapproved, retained and audited for the sake of transparency and compliance. Here’s a deeper look:
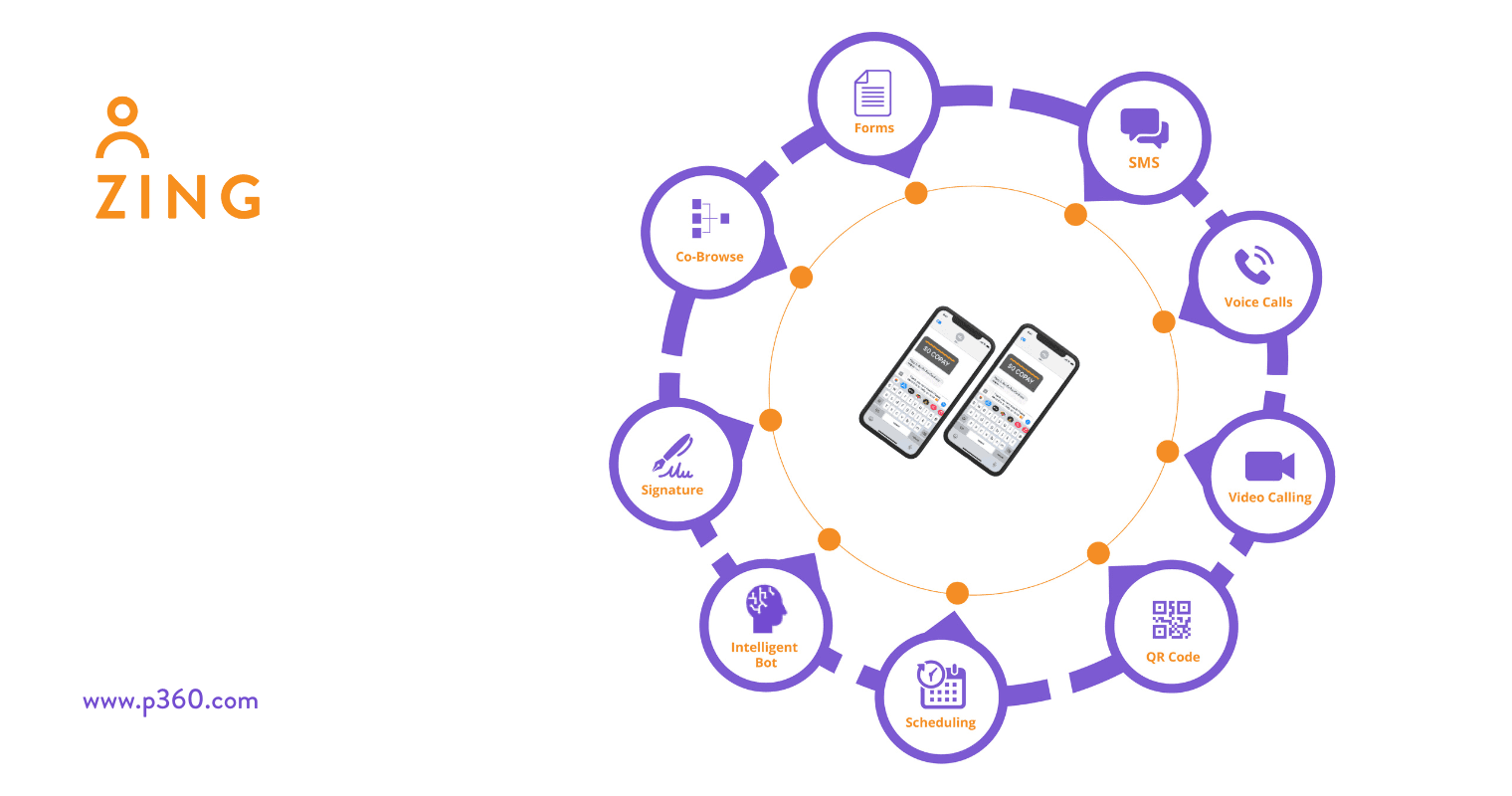
-
Preapproved Messaging
ZING comes equipped with customizable templates that help ensure HCPs don’t receive content they shouldn’t. All messages, digital assets and replies can be easily preapproved by compliance and preloaded into the system. When locked, reps cannot modify any of the templates. They can only choose the HCP and which asset the HCP will receive.
-
AI-Powered Content Moderation
As an added layer of protection, ZING’s built-in, AI-powered content moderation tools help ensure that HCPs never get the wrong message. The system can be programmed to block certain words and phrases. And it can also be used as an added layer of protection for compliance.
-
Contacts Must Be Opted In
Reps aren’t able to just send messages to anyone. They can only be sent to numbers associated with an already established “contact” in the system.
-
Retaining Communications
The new ECCP update underscores the importance of having robust systems in place to retain and produce messages for regulatory inquiries. All of ZING’s data can be stored for appropriate amounts of time (per the client’s guidelines) and can be pulled for legal upon request. This includes messages sent by reps and HCPs, Date/Time, location, browser, operating system, device used and much more.
Final Thoughts
For the pharmaceutical industry, compliant communication with HCPs is essential. However, traditional communication methods like email and phone calls can often be time-consuming and inefficient, leading to missed opportunities. This is where compliance-enabled texting comes in. And ZING is leading the way.
With ZING, pharma reps can send product information, research and other important updates to HCPs quickly and securely. Additionally, HCPs can use this technology to ask questions and receive quick answers from pharma reps without navigating complex email or phone systems. ZING provides all the features you’d expect from an enterprise-grade messaging platform designed with pharma regulations in mind. And now that the compliance argument has been settled, pharmaceutical teams of all sizes can text with confidence with ZING.